
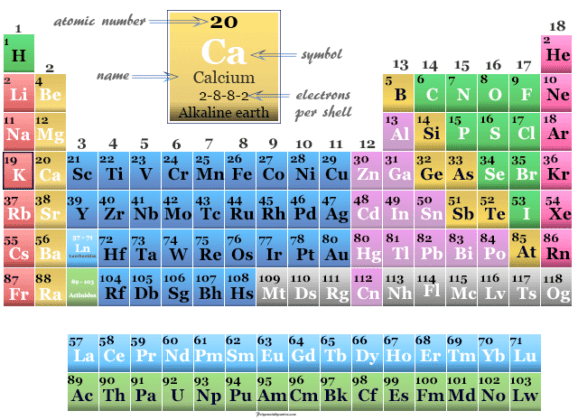
Calcium is the fifth most abundant element by mass (3.4) in both the Earth's crust and in seawater. It is a group 2 metal, also known as an alkaline-earth metal, and no populated d-orbital electrons. Cow milk also contains a large amount of calcium phosphate, which is why human culture encourages children and those particularly susceptible to osteoporosis to drink milk. Calcium is the 20th element in the periodic table. Human bones are made up of mostly calcium phosphate (\(Ca_3(PO_4)_2\)). With the waters rich in sunlight and minerals like calcium, photosynthesis in sea plants is highly favored, allowing fish and other marine life to flourish in these regions. Over massive amounts of time, these calcium deposits grow into gigantic reefs, some of which can be seen from space (like the Great Barrier Reef in Australia). Coral secrete calcium carbonate over the period of their life, then die to allow new coral to build on top of their calcium carbonate structure. One of the most important calcium deposits is in coral reefs, which are comprised of mostly calcium carbonate. In plants, calcium is also important in the cell wall, membrane, and vacuole. Besides skeletal functions, the Ca2+ ion in animals and many organisms also plays an essential role in signal transduction pathways, neurotransmission, muscle function, fertilization, and enzymatic function. Shells of aquatic organisms, snail shells, and egg shells are all composed of mostly calcium carbonate, which can be dissolved in acid. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air.
#CALCIUM NUMBER ON PERIODIC TABLE FULL#
Squares for each element are distorted in proportion to the numerical value of the abundance.\)īecause calcium is essential for life, it can be found in all organisms, living or dead. periodic table, in full periodic table of the elements, in chemistry, the organized array of all the chemical elements in order of increasing atomic number i.e., the total number of protons in the atomic nucleus. 163 languages Tools Calcium is a chemical element with the symbol Ca and atomic number 20. A cartogram depicting the abundance of elements in the earth's crust. In the Modern Periodic Table, calcium (atomic number 20) is surrounded by elements with atomic numbers 12, 19, 21 and 38. This shows up best using the "Bar chart" option on the chart. Notice the "sawtooth" effect where elements with even atomic numbers tend to be more strongly represented than those with odd atomic numbers. The chart above shows the log of the abundance (on a parts per billion scale) of the elements by atom number in our sun. Image showing periodicity of the logarithm of the abundance (by atom rather than weight) in the sun of the chemical elements as a heat map on a periodic table grid. Image showing periodicity of the logarithm of the abundance in the earth's crust of the chemical elements as a heat map on a periodic table grid. Use the links in the location column for definitions, literature sources, and visual representations in many different styles (one of which is shown below) Location Local concentrations of any element can vary from those given here an orders of magnitude or so and values in various literature sources for less common elements do seem to vary considerably.Ībundances for calcium in a number of different environments. Values for abundances are difficult to determine with certainty, so all values should be treated with some caution, especially so for the less common elements. In this table of abundances, values are given in units of ppb (parts per billion 1 billion = 10 9), both in terms of weight and in terms of numbers of atoms. Abundances of calcium in various environments Apatite is calcium fluorophosphate or chlorophosphate. It occurs as limestone (CaCO 3), gypsum (CaSO 4.2H 2O), and fluorite (CaF 2). Calcium is fifth in abundance in the earth's crust, of which it forms more than 3%.
Besides that, it has 19 synthetic, radioactive isotopes with known half-lives 3.

Group 3-12: Transition and Inner transition metals group. Calcium has 20 protons, 20 neutrons and 20 electrons: 21: Scandium has 21 protons, 24 neutrons and 21 electrons: 22. Group 1: Alkali metals group (hydrogen not included) Group 2: Alkaline earth metals group. Naturally occurring Ca is a mixture of 6 isotopes with mass numbers 40, 42, 43, 44, 46 and 48 1, 3. There are total 18 different groups in Periodic table.
#CALCIUM NUMBER ON PERIODIC TABLE FREE#
Calcium metal is never found as the free metal in nature. Calcium is the 20th element in the periodic table. Calcium (pronunciation KAL-see-em 2 ), represented by the chemical symbol or formula Ca 1, is a soft element belonging to the family of alkali earth metals 3.


 0 kommentar(er)
0 kommentar(er)
